A key challenge being faced by the Revised National Tuberculosis Control Programme(RNTCP)is the long treatment period endured by the patients, which often leads to poor treatment adherence bypatients. This may lead to drug resistance. India has a large and expanding population of MDR-TB patients. New TB drug regimens with a combination of multiple new drugs are the need of the hour. A shorter, safer and more effective regimen is needed to treat both drug sensitive and drug resistant TB. This may increase the adherence and reduce further amplification of drug resistance. Numerous new and repurposed anti-TB drugs have emerged, offering the opportunity to develop shorter, multi-drug, non-toxic ’Universal TB Regimen’, that pre-empts the threat of MDR-TB entirely.
There are nine anti-TB drugs in advanced phases of clinical development for the treatment of drug-susceptible, drug-resistant TB and latent TB infection (LTBI).Of these, six are new and three are approved or repurposed. The six new compounds are Bedaquiline, Delamanid, PBTZ169, Pretomanid, Q203 and Sutezolid, and the three approved or repurposed drugs are Rifampicin, Rifapentine and Linezolid. Several studies evaluating shorter regimens for LTBI are also being undertaken.All these new drugs will offer an enormous opportunity to engage Indian pharmaceutical industry in research partnerships in a sustained and structured way.
Innovative models needed for TB drug development are urgently required.The standard drug development process requires that each new drug is evaluated and approved separately, before it is tested in combination with other compounds. As a result, regulatory approvals for a new combination for TB therapy may take years before it is introduced to patients.
ITRC along with partners will undertake research to evaluate the use of these newer drugs in combination therapy to reduce the duration of therapy.It will focus on establishing and improving the current framework and infrastructure for development of new drugs and combination regimens. A novel strategy would be adopted by advancing the innovative drug development tools such as data standards, data platforms, biomarkers, clinical endpoints, and disease progression models. TB drug candidates in combination will be evaluated and the regulatory science, infrastructure, and diagnostic data required to support this strategy would be developed. It will also help in initiating and sustaining all patients on appropriate anti-TB treatment wherever they seek care.This will encourage information sharing and collaborations to innovate and accelerate TB drug development and get important new therapies to patients.ITRC will also support organizations and stakeholders in accelerating procurement of and access to new TB drug therapies for patients.

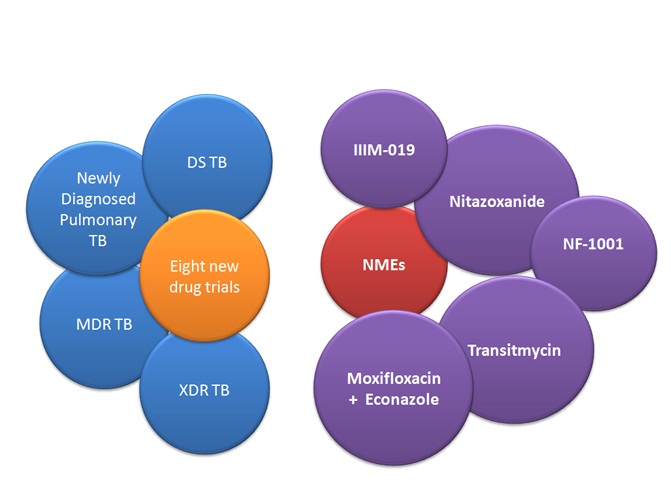
After a detailed landscape analysis, eight key trials are being taken forward for both drug sensitive and drug resistant TB patients. The process of identifying sites for these trials across Indiais currently underway.
Click here to view the Therapeutics Research Portfolio
ITRC: India TB Research Consortium