There is a critical need for new TB vaccines that are more effective than the Bacille Calmette-Guérin (BCG) vaccine in preventingpulmonary as well as extra pulmonary forms of TB in all age groups. The BCG vaccine, which is now over 100-years old, has been shown to be immunoprotective against extra pulmonary forms of TB in children, including TB meningitis. However, the protection provided against pulmonary TB in adults is variable. New vaccines are also required keeping in view the slow decline in TB incidence globally and the persistent threat of MDR-TB.
The pipeline of vaccine includes recombinant BCGs, whole-cell derived vaccines, recombinant viral-vectored platforms, protein and adjuvant combinations, and mycobacterial extracts. For instance, sixteen different TB vaccine candidates are currently in clinical trials globally, with a handful approaching or currently in proof-of concept (Phase IIb) studies in the field, and many more in preclinical development. There are currently eight vaccines in Phase II or Phase III trials.These vaccines aim either to prevent infection (pre-exposure) or to prevent primary progression to disease or reactivation of Latent TB Infection(LTBI -post-exposure,). These would have a critical role in India’s fight against TB.
All vaccine research undergoing in different Indian academic labs will be evaluated and promising vaccine leads will be developed further to advance through manufacturing, toxicity and clinical trials.
ITRC will help design, conduct and validate new vaccine candidates in India. Itwill also support the development of indigenous vaccine candidates through institutional collaboration and funding.


The vaccine candidates under clinical development by different manufacturers will beevaluated for immunogenicity and efficacy in the Indian population. Based on the different clinical end points various TB vaccines with different targets/ clinical end-points will need to be evaluated in Indian population and these are:
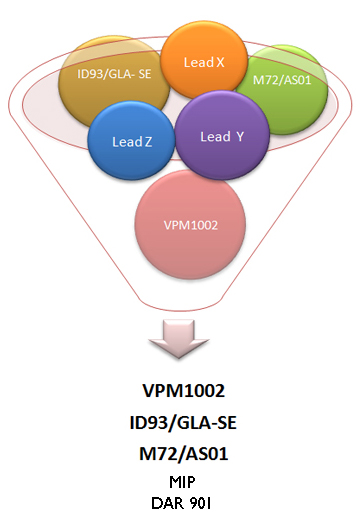
The landscaping of TB vaccines has identified three vaccine candidates which can be tested for different end points in the immediate future.
The three vaccines identified are:
Depending on the clinical end point of the vaccine, different target populations will have to be recruited in various clinical trials to test respective vaccine efficacy.Investigational New Dossiers (IND) will be submitted to regulatory authorities for approval by Drug Controller General of India. All investigational vaccines will be released by CRI, Kasauli after testing and trial site ethics committee will approve participation of each site.
Click here to view the Vaccines Research Portfolio
ITRC: India TB Research Consortium