
The Government of India is committed to extend healthcare services to its 1.38 billion population as part of India’s Universal Health Coverage (UHC) agenda. The National Health Policy has recommended an increase in public spending on healthcare services from existing 1.15 percent to 2.5 percent of GDP. It notes that this can significantly reduce the out-of-pocket-expenditure of the overall healthcare spending. With such a challenge, it is essential for the government to ensure optimal utilization of existing resources to ensure that the greatest amount of health is generated for every rupee spent. Therefore, Department of Health Research has established a mechanism for Health Technology Assessment for evaluation of appropriateness and cost effectiveness of available and new health technologies in the country as part of research governance mandate of the Department. HTA is a multidisciplinary process that summarizes information about the medical, social, economic and ethical issues related to the use of a health technology in a systematic, transparent, unbiased, robust manner. Its aim is to inform the formulation of safe, effective, health policies that are patient focused and seek to achieve best value. In essence, HTA is a process which systematically evaluates health interventions to ensure they represent good value for money.
Health Technology Assessment in India (HTAIn) is a sub-scheme under the umbrella scheme Human Resource and Capacity Building in the 15th Financial Commission approved for year 2021-22 to 2025-26 under the Department of Health Research (DHR), Ministry of Health & Family Welfare (MoHFW), Government of India to facilitate the process of transparent and evidence-informed decision making in the field of healthcare. HTAIn is entrusted with the responsibility to analyses health technologies viz. medicines, devices and health programmes for its cost-effectiveness, clinical-effectiveness and equity issues by means of Health Technology Assessment (HTA), and in turn help in decision making for an efficient use of the limited health budget and provide people access to the quality health care reducing their out of pocket expenditures (OOPs) on health.
The need to establish a Medical Technology Assessment Board (MTAB) was recommended by 12th Plan Working Group on Health Research and also by the Planning Commission (now NITI Aayog) in its 12th Plan document on Social Sector. The Standing Committee of Parliament in its 56th Report on the Examination of Demand for Grant of DHR for the year 2012-13 has emphasized early setting up of MTAB. The National Health Policy, 2017 has also highlighted the importance of HTA by stating ‘One important capacity with respect to introduction of new technologies and their uptake into public health programmes is health technology assessment.
Established in 2017, HTAIn has extended support to various verticals (e.g Maternal Health, Child Health etc.) of the health ministry at the center and also at state level, in evidence-based decision making.
Health Technology Assessment in India (HTAIn) consists of an in-house HTAIn Secretariat, Board, Technical Appraisal Committee (TAC), Regional Resource Hubs/ Centres (RRCs) and Technical Partners (TPs).
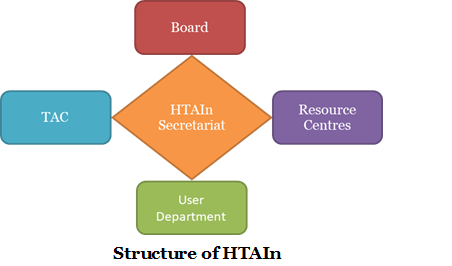
HTAIn Secretariat is a DHR- in-house body that coordinates between the User Department, TAC, Technical Partners and Resource Centres. Secretariat consist of Scientists, Economists, Health Policy Analyst, Financial Consultants, Programme Manager, Data Entry Operators and Multi-Tasking Staffs. It provides necessary assistance to the Technical Partners (TPs) and Resource Centres (RRCs) wherever required and monitor the progress of studies being conducted. Secretariat may also undertake topic(s) to study in certain situations. Besides that, secretariat conducts all the TAC and Stakeholders consultation meetings in DHR and ensures transparency at all stages of the study by consultation and regular updates from the Technical Partners and Resource Centres.
HTAIn Board was set up in 2017, to take final policy decisions on the recommendations of HTA studies. The Board consist of Policy-Makers, Clinicians, Bureaucrats and Experts from different Government Bodies (Central as well as States) etc. Board is the highest decision-making body of HTAIn that endorses/ appraise the TAC approved Outcome Reports/ Recommendation. The Board may also look into the gaps in evidence and instruct for further research. i.e. Board can identify the area that require further research.
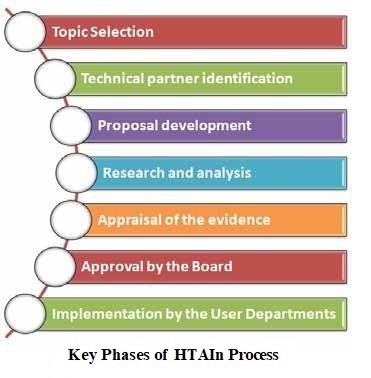
Technical Appraisal Committee (TAC) is a multidisciplinary body with experts drawn from different areas viz economists, clinicians, researchers, social scientists, health policy experts etc. There may be co-opted members in the TAC depending upon the study under consideration by HTAIn. The Committee is invariably headed by an eminent person. It ensures the appraisal of the study at different stages viz. support in analyzing feasibility of topic for HTA, allocation, proposal development, outcome report and recommendations. TAC does the quality assurance and provides overall stewardship to the HTAIn. Till 31st December 2021, twenty-Six (26) TAC meetings have taken place in DHR for the appraisal of the HTA proposals/ Outcome Reports submitted by the TPs/ RRCs and discussing potential challenges HTAIn may face in the Indian scenario such as perspective, equity issues, availability of evidences, etc.
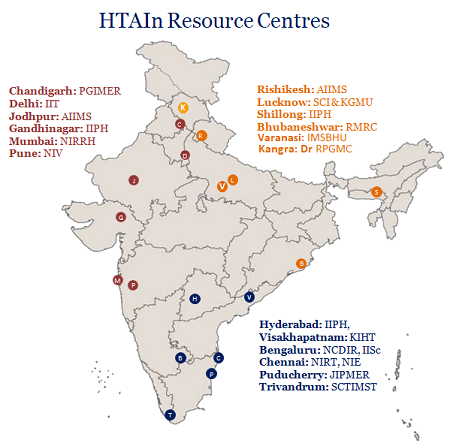
Regional Resource Centres have been established in Government research institutes entrusted with the responsibility to conduct HTA and other multi-centric studies allocated by HTAIn Secretariat. They are identified on the basis of their capacity and previous experience in HTA. DHR provides requisite manpower support to the Regional Resource Centres or Resource Centres/ hubs in order to bridge the gap between Central and the State Governments, assist capacity building, support States located in the vicinity and undertake the studies allocated to them by the Secretariat. The mentor of the Centres liaise with the officials of the State Governments and sensitize them about a need for Health Technology Assessment (HTA) for any health intervention. 16 institutes were already identified to establish the Regional Resource Centres of HTAIn till the year 2020-21 and one more Resource Centre was established in IISc. Bangalore in 2021-22. All the 17 Resource Centres of HTAIn are as follows:
Stakeholders of HTAIn include the user department e.g. Central/ State Govt., NHM, RSBY or NPPA, public health authorities, policy makers, medical insurers, regulatory agencies, industrial associations (e.g. manufacturers, suppliers, wholesalers, distributors and retailers), academicians or methodological experts, researchers, social groups, NGOs, patient group and so on. Stakeholders are individuals, organizations or communities that have a direct interest in the process and/or outcomes of the study under consideration by the HTAIn, therefore their participation is required to maintain the integrity of HTAIn and for a wider acceptance of its recommendations. Thus, a stakeholder’s consultation meeting is called after completion of every study to take their feedback and inputs. Outcome reports are also uploaded on the website for the feedback and suggestions. Conflicts of interests, if any, are addressed making the process transparent.
The logo of Health Technology Assessment in India (HTAIn) is in the form of a shield which represents the protecting role of HTAIn towards its citizens from the financial hardships arising out of health care seeking. The top of the shield is marked with Ashok Chakra, depicting the allegiance of HTAIn towards the constitutional values and the nation. The Rod of Asclepius and the symbol of the Indian Rupee are placed side by side below the National Emblem; as while making a decision about the cost-effectiveness of an intervention, HTAIn gives due consideration to both public health potential and the costs associated with the intervention. “ सर्वेः सन्तु निरामयाेः” is scripted in Devnagri script on a ribbon, which means “Let all be healthy”, and expresses the devotion of HTAIn towards the values of Universal Health Coverage (UHC).
